|
NOVIDADES
No, this article is not about the liquid metal T-1000 from the Terminatormovies – but if we can interest you in some more down to earth leading-edge research, keep reading nevertheless. The conventional definition of liquid metals refers to metals with melting points near or below room temperature. Liquid metals are elemental or alloyed materials predominantly composed of post-transition and zinc group metals (excluding zinc itself). Mercury (Hg) and gallium (Ga) are the two most recognized elemental liquid metals. Hg has a low melting point of -38.8°C, but its potential hazards rule it out for many applications. Ga has a melting point of 29.8°C and is considered to have low toxicity, which makes it suitable for many applications. In a recent review in ACS Nano ("Emergence of Liquid Metals in Nanotechnology"), the authors extend the group of liquid metals somewhat subjectively to those with relatively low melting points under 300°C. As they explain, this is based "on the accessibility of achieving a molten state, considering that even cookware in household kitchens can operate within this range of temperatures. There are several other reasons for choosing this limit: 1) many industrial processes operate below this limit, namely, polymer melt processing, thus opening the possibility of new processes for nanocomposites; 2) common laboratory equipment such as ovens and hot plates operate in this range; and 3) liquid metals may be co-processed with other liquids, such as water or organics, that are routinely processed at increased temperatures." The recent interest in liquid metals is based on the properties that differentiate them from common liquids such as water or organics. In addition to their chemical reactivity, the electronic behavior of these liquids, combined with the strong interatomic interactions throughout the bulk, lead to liquids with high densities, thermal and electrical conductivities, and optical reflectivity (over a wide range of wavelengths). Alloying liquid metals with other elements provides additional depth to the field of liquid metals by creating materials with tunable properties. Although metallurgy of solids is a mature field in many aspects, the study of low-melting-point metals is still underexplored, especially when dealing with nanoscale domains of solids within liquid alloys. This presents nanotechnology researchers with new potential approaches for the synthesis of nanomaterials and investigations of fundamental physics and chemistry at small length scales. Fundamentally, liquid metals are reaction media with great potential for the synthesis and manipulation of a variety of nanomaterials within the bulk or on the surfaces of liquid metals. 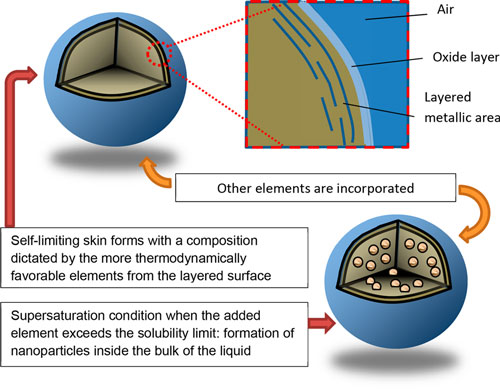 Schematics depicting how low-dimensional materials can be formed using liquid metals: (top) metallic layering near the interface of liquid metal and (bottom) nucleation of solids after supersaturating the liquid core with the added element. (Reprinted with permission by American Chemical Society)
They also note that the resulting change of density from the bulk to the liquid-vapor interface "causes a transition from a liquid metallic state (with nearly free electrons) to a more ordered state at the surface. This change results in surface “layering” in many liquid metals. These layers are a few atoms thick. Although these layers are still liquid-like, they have increased atomic order (relative to the bulk liquid) in liquid metals such as Ga and Sn." Of particular interest for the fields of nanotechnologies are the potential applications of liquid metals as nanomaterials. These applications includes drug delivery, catalysis, composites, dynamic surfaces and structures, solders, conductive inks, and plasmonic structures. 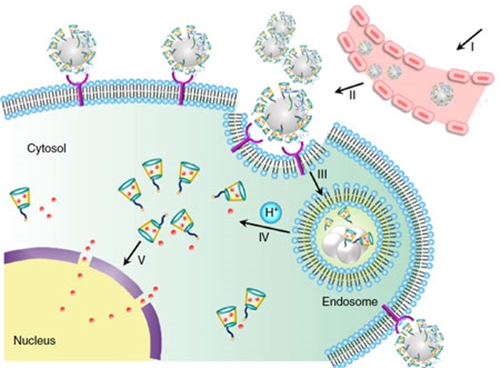 Schematic design of a transformable liquid-metal drug delivery system. pH-responsive delivery of Dox by LM-NP/Dox-L to the nuclei for the targeted cancer therapy. Accumulation of LM-NP/Dox-L at the tumour site through passive and active targeting; II) specific binding to the overexpressed receptors on the tumour cells; III) receptor-mediated endocytosis; IV) acid-triggered fusion of LM-NP/Dox-L and endosomal/lysosomal escape of Dox-containing ligands; V) accumulation of Dox in the nucleus. (Reprinted with permission by Springer Nature from "Transformable liquid-metal nanomedicine", Nature Communications, doi: 10.1038/ncomms10066)
Although the optimal mechanisms for controlling such dynamic particles remain underexplored, in summary, liquid metal particles have many unique physical properties relative to solid metal particles that present opportunities for new research paths. "Liquid metals, which can be rendered into nanoparticles readily, have properties that can be tuned based on the addition of other materials into the bulk or on the surface of the liquid metal," the authors conclude their review. "The combination of fluid and metallic properties, along with reactivity, processability, field responsiveness, and tunable properties, suggests that the field of liquid metals is ripe with opportunities for nanotechnology." By Michael Berger. Posted: July 11, 2019. |
|||||||||||||||||||||||||