|
NOVIDADES
Currently, a key issue hindering the utility of nanoparticles in more sophisticated applications is reproducibility. This problem is, however, partially intrinsic, as the product of synthesis is always prone to yield a polydispersion of nanoparticles, sometimes with a broad distribution of sizes, shapes, and defects. This makes nanoparticle characterization a crucial step required to fully comprehend the origin of nanoparticle behavior, and subsequently translate their performance benefits from laboratories into specific real word applications. There is at the same time a need to establish standardized, well-characterized nanoparticles that vary in size, shape, durability, composition and surface reactivity for use in metrology, characterisation, exposure and hazard assessment (notably for comparative benchmarking purposes in toxicology). Researchers are still challenged by the task of determining the physicochemical properties of nanoparticles and exploring their structure–function relationships. A key limitation is their ability to fully investigate the nanoscale realm: Different characterization techniques are based on different physical properties, therefore only providing a partial picture of the nanoparticle characteristics. Making matters more challenging yet, the characterization methods themselves can directly affect the measured quantities of nanoparticles. Nanoparticle characterization is a broad and complex discipline. However, measurement and standardization often lag behind the rapid development of new nanomaterials and their applications. Furthermore, the demands on reproducibility and quality control increase steadily as new materials leave the discovery stage and move into commercial applications. Nanoparticles exist in various chemical compositions ranging from micelles to metal(oxide)s, from synthetic polymers to large biomolecules. Each of these materials features a completely different chemistry, which can be analyzed by a variety of methods including optical spectroscopy, X-ray fluorescence and absorbance, Raman spectroscopy, and solid-state NMR. However, often the behavior of nanoparticles is largely governed by their nanometer dimensions. As such, throughout nanoparticle characterization, the investigation of size, shape, surface charge and porosity is a fundamental step for fully understanding and predicting their behavior. These essential parameters are the focus of a review article in Advanced Materials ("Nanoparticle Characterization: What to Measure?"). It provides a set of guidelines to investigate and characterize the key parameters defining a nanoparticle sample, namely size, shape, surface charge, and porosity. First, the authors define these physicochemical terms and their implication in affecting nanoparticle properties. They then provide a critical overview of established and specialized techniques currently used for evaluation of nanoparticles, and discuss their practical advantages and disadvantages. Concluding their review, the authors propose some reasonable recommendations of how the physicochemical parameters of nanoparticles should be investigated, and how to characterize these key properties in different environments according to the intended nanoparticle use. The review provides a description of these physicochemical terms in the context of nanoparticle technology, and the different physical and experimental means in which these parameters can be defined. Size and shape affect the nanoparticle functionalization capacity, fluid drag and diffusion, optical properties, and uptake into cells. 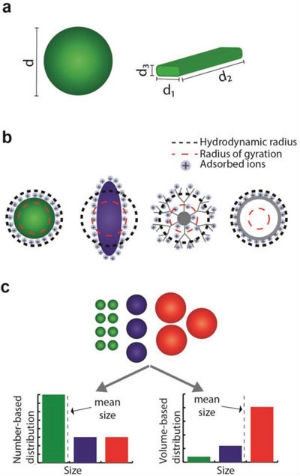 Nanoparticle size characterization. a) Spherical particles are described by a single size parameter. However, for nonspherical nanoparticles, several dimensions are needed to fully report their dimensions; b) the calculation of the effective radius is based on nanoparticle behavior or on the method of detection. The definition of this quantity might differ considerably from the physical nanoparticle dimensions; c) different mean sizes can be calculated for a nanoparticle population according to the weighting factors assigned to the population components. (Reprinted with permission by Wiley-VCH Verlag)
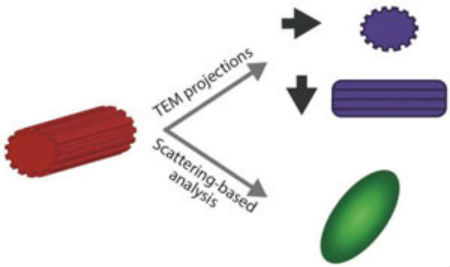 Nanoparticle shape characterization. TEM provides nanometer resolution on nanoparticle morphology. However, the measured shape is a 2D projection of the nanoparticle morphology, and therefore depends on the relative orientation of the nanoparticle and the electron beam. Scattering-based techniques provide qualitative information on the nanoparticle shape. (Reprinted with permission by Wiley-VCH Verlag)
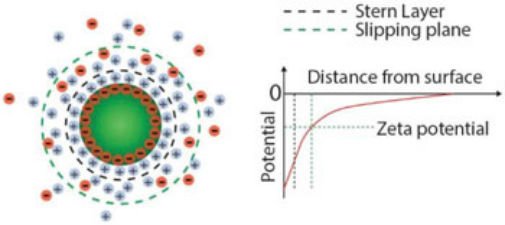 Surface charge and zeta potential of nanoparticles in suspension. Charges on the nanoparticle surface are screened by the free ions in solution, giving rise to two ion layers: a first layer of adsorbed ions on the nanoparticle surface, the so-called Stern layer; a second layer of stationary but diffusing ions that move with the particle. The zeta potential is defined as the potential difference between the slipping plane, i.e., the plane that “separates” the cloud of stationary ions around the particles from freely diffusing ions in solution, and the potential of the bulk solution. (Reprinted with permission by Wiley-VCH Verlag)
In addition, porous or hollow nanoparticles also exhibit a vast internal surface area, which can be further functionalized to impart additional functionalities, such as the design of smart nanoparticle drug delivery systems. The possibility of synthesizing nanoparticles featuring porous frameworks – such as metal-organic frameworks (MOFs) – has greatly expanded the range of application of nanomaterials. Porosity provides the nanoparticles with a drastic increase in their surface-to-volume ratio, which can exceed that of solid particles with equal dimensions by several orders of magnitude. They also introduce some specialized techniques for nanoparticle characterization, which enable to expand the accessible range of information to gain deeper insights into specific nanoparticle properties. Given the large number of existing approaches and techniques, including the combination of different methods, different variations of the same techniques, and different approaches to data analysis for a same technique, the authors caution that their review cannot provide an exhaustive list of all available methods for nanoparticle characterization. Rather, they provide a selection of methods that in their opinion are best suited to characterize a broad range of nanomaterials, which are commonly used and well established: – Scanning Electron Microscopy (SEM) – Atomic Force Microscopy (AFM) – X-Ray Diffraction – Small-Angle X-Ray Scattering – Mass Spectrometry – Gas Sorption – Static Light Scattering – Nanoparticle Tracking Analysis – Electrophoretic Light Scattering – Analytical Ultracentrifugation – Size Exclusion Chromatography – Analytical Ultracentrifugation – Fluorescence Correlation Spectroscopy – Tunable Resistive Pulse Sensing – Electron Cryo-Microscopy – Electron Tomography – NMR Cryoporometry and DSC Thermoporosimetry – Super-Resolution Microscopy – Single Molecule Fluorescence Microscopy – Nanomechanical Resonators 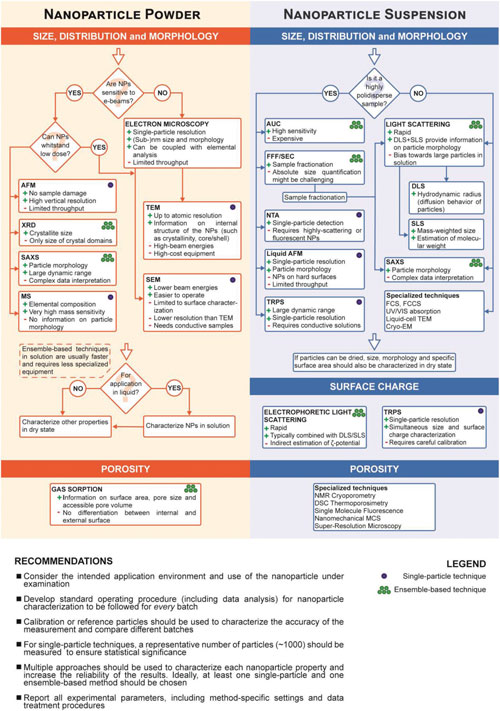 Flowchart depicting the main steps for the characterization of size, size distribution, morphology, surface charge, and porosity of nanoparticles (NP), both in dry state and in solution.
To be able to correlate the physicochemical properties of the nanoparticles with their performance in a specific task, characterization needs to be both accurate and precise. Therefore, as the authors recommend, standardized standard operating procedures should be developed to improve the comparability of results between different materials and laboratories: "It is crucial that new data can be compared and evaluated with previously published results in a meaningful way. This will require conclusive and harmonized analytical protocols to be applied worldwide." Concluding their review, the authors make the following general recommendations for nanoparticle characterization: 1. Consider the intended form the target application of the nanoparticles. The form (colloidal suspension or solid powder) often dictates the need for the specific characterizations that should be used. 2. Place reasonable bounds on the precision and statistical confidence with which physicochemical properties need to be measured to satisfy the needs of the application. For example, the statistical demands in the quality control for pharmaceutical formulations will typically be higher than for industrial coating materials. 3. Develop or adopt standard characterization and standard operating procedures to be systematically and thoroughly followed for every batch. 4. The protocol used to measure the physicochemical parameters as well as the meta data should be described in detail. Besides a detailed description of the experimental protocol, the experimental parameters, and method-specific settings that were used, this should include how the data were analyzed (e.g., what model was used to obtain a certain value). 5. In the case of suspensions, the exact chemical composition of the liquid matrix should be specified. Both pH and ionic strength should be measured and reported for aqueous solutions. 6. Measured parameters should be calibrated against a standardized reference. If no standardized references for a quantitative comparison with the nanomaterial at hand are available, internal references can be used to, at least, ensure consistency between batches. For example, obtaining similar size distributions from different nanoparticle batches and/or a constant reference sample should ensure the reproducibility of results. 7. Whenever feasible, more than one technique should be used to characterize the same quantity. 8. The obtained results should be compared with published data whenever feasible. By Michael Berger. NW. Posted: June 05, 2019. |
|||||||||||||||||||||||||