|
NOVIDADES
A novel, pot-shaped, carbon nanomaterial developed by researchers from Kumamoto University, Japan is several times deeper than any hollow carbon nanostructure previously produced. This unique characteristic enables the material to gradually release substances contained within and is expected to be beneficial in applications such as drug delivery systems. 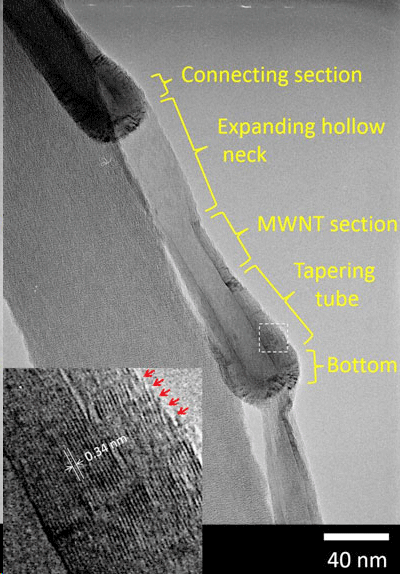 Carbon nanopots are made of several different laminated graphene layer structures as labeled by the yellow text. The magnified image (inset) reveals the area indicated by the dashed line in the main image. The numerical value in the inset is the average value of layer spacing, and the arrows indicate graphene edges exposed on the outer surface of the body. (© Materials Research Society, Dr. Eng. Hiroyuki Yokoi)
Why are these tiny substances needed? One reason is that reactions with other materials can be much larger if a substance has an increased surface area. When using nanomaterials in place of existing materials, it is possible to significantly change surface area without changing weight and volume, thereby improving both size and performance. The development of carbon nanomaterials has provided novel nanostructured materials with shapes and characteristics that surpass existing materials. Now, research from the laboratory of Kumamoto University's Associate Prof. Yokoi has resulted in the successful development of a container-type carbon nanomaterial with a much deeper orifice than that found in similar materials (Journal of Materials Research, "Novel pot-shaped carbon nanomaterial synthesized in a submarine-style substrate heating CVD method"). 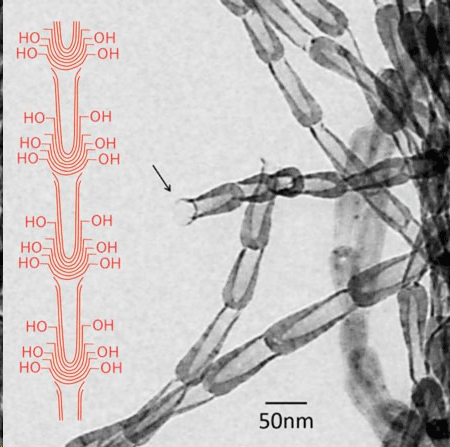 The black arrow indicates the end of the opening of carbon nanopot. A structural schematic of the carbon nanopot showing hydroxyl groups bonded to the edges of the graphene layers near the closed end of the nanopot is also indicated (not to scale). (© Materials Research Society) To create the new material, researchers used their own, newly developed method of material synthesis. The container-shaped nanomaterial has a complex form consisting of varied layers of stacked graphene at the bottom, the body, and the neck areas of the container, and the graphene edges along the outer surface of the body were found to be very dense. Due to these innovate features, Associate Prof. Yokoi and colleagues named the material the "carbon nanopot." The carbon nanopot has an outer diameter of 20 ~ 40 nm, an inner diameter of 5 ~ 30 nm, and a length of 100 ~ 200 nm. During its creation, the carbon nanopot is linked to a carbon nanofiber with a length of 20 ~ 100 µm meaning that the carbon nanopot is also available as a carbon nanofiber. At the junction between nanopots, the bottom of one pot simply sits on the opening of the next without sharing a graphene sheet connection. Consequently, separating nanopots is very easy. "From a detailed surface analysis, hydrophilic hydroxyl groups were found clustered along the outer surface of the carbon nanopot body," said Associate Prof. Yokoi. "Graphene is usually hydrophobic however, if hydroxyl groups are densely packed on the outer surface of the body, that area will be hydrophilic. In other words, carbon nanopots could be a unique nanomaterial with both hydrophobic and hydrophilic characteristics. We are currently in the process of performing a more sophisticated surface analysis in order to get that assurance." Since this new carbon nanopot has a relatively deep orifice, one of its expected uses is to improve drug delivery systems by acting as a new foundation for medicine to be carried into and be absorbed by the body. Kumamoto University, Posted: Aug 05, 2016. |
|||||||||||||||||||||||||